Biological Science 2015/11/19
Kohji Yamada, Misuzu Yamashita-Yamada, Taishi Hirase, Tadashi Fujiwara, Kenichi Tsuda, Kei Hiruma and Yusuke Saijo
DOI 10.15252/embj.201591807
The EMBO Journal (2015) embj.201591807
In plants, when a central mediator of cell surface immune receptors is depleted by pathogen attack, a danger-sensing signaling pathway becomes engaged in defense activation.
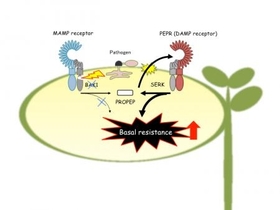
Plants employ an assembly of cell surface receptors to recognize and respond to invasion by potentially infectious microbes. Detection of microbe-associated molecular patterns (MAMPs, e.g., bacterial flagellin protein and fungal cell wall components) and endogenous danger-associated molecular patterns (DAMPs) by these immune receptors leads to an enhanced state of immunity. Pathogens infect a host by overcoming an initial defense layer induced by MAMP receptors. Despite this suppression, MAMP receptor-triggered signaling is linked to basal resistance that restricts pathogen growth. In the reference plant Arabidopsis thaliana, endogenous small peptides called PROPEPs expose elicitor-active Pep epitopes to cell surface Pep receptors (PEPRs), thereby serving to propagate defense signaling triggered by MAMP receptors. However, it remains unclear at a molecular level how PROPEPs (or their derivatives) are released to elicit PEPRs, as well as how this putative DAMP signaling is coordinated with MAMP signaling, in response to pathogen challenge.
The Saijo Lab at NAIST, led by Yusuke Saijo, formerly of the Max Planck Institute for Plant Breeding Research, Cologne, Germany, has investigated how DAMP sensing and signaling contributes to the control of plant immunity.
In plants, leucine-rich repeat (LRR) ectodomain-containing receptors represent a major class of cell surface receptors that monitor the extracellular space for a wide range of ligands, including MAMPs and DAMPs. The Arabidopsis LRR-receptor kinase (RK) BAK1 physically associates and functions with different LRR receptors, and thus represents a central regulator of plant immunity. Indeed, bak1-knockout plants exhibit severe defects in a major branch of MAMP-triggered immunity, including the pathway mediated by LRR-RK FLS2 that recognizes bacterial MAMP flagellin. In their study, Kohji Yamada et al. sought to identify the mechanisms by which plants activate effective resistance in response to or in the presence of pathogen assaults on BAK1.
PEPR1/PEPR2 per se are also among the BAK1-associated LRR-RKs: early PEPR-mediated outputs (e.g., bursts of reactive oxygen species or ethylene production) are reduced in bak1-knockout plants, consistent with the established view that BAK1 acts as a shared coreceptor to mediate signaling from partner LRR-RKs. Unexpectedly, however, Saijo's team found that loss of BAK1 shifts the balance of PEPR-mediated signaling to promote cell death. In this respect, BAK1 was previously shown to repress cell death via a process that remains poorly understood. The present study reveals that the PEPR pathway defines one of the pro-death pathways that are negatively regulated by BAK1. The team then profiled PEPR-regulated genes whose expression was influenced by bak1 mutation. They found that in the absence of BAK1, the balance of PEPR-mediated signaling outputs is rewired between the defense-related phytohormones salicylate and jasmonate: salicylate (SA)-based defenses are strengthened, whereas jasmonate (JA)-based defenses are weakened. Using bak1 and bak1 pepr1 pepr2 mutant plants, they verified that PEPRs are required for basal resistance to virulent pathogens in the absence of BAK1. Moreover, to gain insight into how PROPEPs provide elicitor-active ligands during pathogen challenge, the team generated transgenic plants expressing a PROPEP3-Venus (a GFP variant) fusion protein driven under the control of its native regulatory DNA sequences. Their results reveal that, following initial MAMP-dependent induction, pathogen-injected virulence-promoting effectors and cell death associated with bak1 disruption additively enhance the production and release of the PEPR proligand PROPEP3. These observations provide, for the first time, compelling evidence that the PEPR pathway mediates DAMP sensing and signaling in plant immunity.
To ensure the biological significance of the findings obtained in Arabidopsis bak1-knockout mutants, the team further investigated various pathogens that may deplete BAK1 during infection. They found that invasion by the phytopathogenic fungus Colletotrichum higginsianum, which causes anthrax disease in crop plants, leads to selective depletion of BAK1. Their results indicate that BAK1 depletion by the fungus contributes to fungal invasion, but at the same time stimulates PEPR-mediated post-invasion defenses. Therefore, their work provides evidence that PEPR-mediated DAMP signaling retains plant immunity when the initial MAMP-triggered defenses are compromised by BAK1 depletion, and also demonstrates the potential usefulness of the PEPR pathway as a tool for increasing resistance of crop plants to pathogens such as anthracnose fungi.
Dr. Yusuke Saijo is based at the Plant Immunity Laboratory, Graduate School of Biological Sciences, Nara Institute of Science and Technology, Ikoma, Japan.