Materials Science 2017/05/12
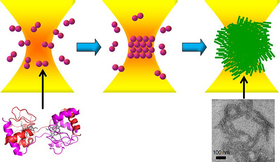
Protein amyloids receive much attention due to their correlation with serious diseases and their promising mechanical and optical properties as future materials. Amyloid formation has been conducted by tuning temperature and chemical conditions, so that its nucleation and the following growth are analyzed as ensemble dynamics. Here we succeeded in generating a single spherical assembly consisting of amyloid fibrils of cytochrome c domain-swapped dimer by laser trapping. The amyloid fibrillar structure was confirmed by fluorescence characterization and electron microscopic observation. The prepared spheres are further manipulated individually in solution to fabricate a three-dimensional micro-structure and a line pattern. Amyloid formation dynamics and amyloid-based micro-structure fabrication are demonstrated based on direct observation of a single spherical assembly. Laser trapping will thus play important roles in studying fundamental dynamics and mechanism of amyloid formation and in designing novel bottom-up nanomaterials from amyloids.
【Paper title and author(s)】
- Title: A Single Spherical Assembly of Protein Amyloid Fibrils Formed by Laser Trapping
- Author(s): Ken-ichi Yuyama, Mariko Ueda, Satoshi Nagao, Shun Hirota*, Teruki Sugiyama*, Hiroshi Masuhara* (*Corresponding Authors)
- Publication: Angewandte Chemie International Edition "Hot Paper
- DOI: 10.1002/ange.201702352