Materials Science 2014/10/16
Hydrogenase catalyzes the reversible cleavage of H2 (Figure 1). It is one of the most highly studied enzymes, with promising applications in future energy technologies. The collaborative research group of Prof. Shun Hirota and Assist. Prof. Hulin Tai at Graduate School of Materials Science, Nara Institute of Science and Technology (NAIST; President, Naotake Ogasawara) and Prof. Yoshiki Higuchi at University of Hyogo (President, Masayoshi Kiyohara) measured the FT-IR spectra of nickel-iron hydrogenase under light irradiation at low temperature. They found that the redox state of the site called "iron-sulfur cluster" controls the reversible catalytic decomposition and synthetic production of H2 in nickel-iron hydrogenase, and the proximal iron-sulfur cluster functions as the on/off switch for its catalytic reaction. These finding may be useful for development of new fuel cells and hydrogen synthetic catalysts.
【 Related Link 】
The paper is published in the Journal below.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201408552/abstract
Here is the bibliographic information of the paper.
Hulin Tai, Koji Nishikawa, Masayuki Suzuki, Yoshiki Higuchi, Shun Hirota, Control of the Transition between Ni-C and Ni-SIa States by the Redox State of the Proximal Fe-S Cluster in the Catalytic Cycle of [NiFe] Hydrogenase. Angewandte Chemie International Edition 50, 13817-13820 (2014).
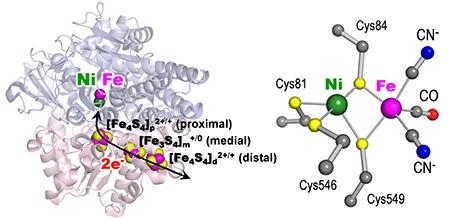 Figure 1. Molecular (left) and active site (right) structures of nickel-iron hydrogenase.
Figure 1. Molecular (left) and active site (right) structures of nickel-iron hydrogenase.




